Rehabilitation Technology and Design
Exercise Equipment: Emphasizing Usability, Safety, and Effectiveness
The latest DAVID Medical-certified G-Line, featuring automatic weight selection, is renowned for its exceptional biomechanical precision, isolated movement capabilities, and the incorporation of a Natural Resistance Curve. Combined with the Nordic Design aesthetics, DAVID stands as the epitome of best-in-class Exercise Equipment.
Safety is Paramount
At DAVID, safety goes beyond the typical considerations in CE-certification. While most devices today meet safety standards, we prioritize safety by ensuring that training yields positive results for your body, free from negative effects such as strained muscles and hurting joints.
Efficiency Matters
Effectiveness remains a core objective in our equipment design. Within safety limitations, all exercises are designed to be as effective as possible. This dedication has driven our involvement in and initiation of research across various areas for a remarkable quarter of a century.
User-Friendly Experience
Our third objective revolves around providing a positive user experience with our technology. DAVID Exercise Equipment is designed to be exceptionally user-friendly, empowering anyone to use it without external assistance once initial guidance and instructions are provided.
Experience the Difference
With DAVID Exercise Equipment, you gain access to an exceptional blend of usability, safety, and effectiveness. As pioneers in the industry, we continue to push boundaries and elevate the training experience, ensuring that each session with our equipment is both enjoyable and beneficial. Discover the DAVID difference for yourself today.
The rehabilitation technological principles explained
Biomechanical precision
Biomechanical engineering ensures safety, control and effectiveness. This means that optimally designed device can be utilized both in early stage rehabilitation as well as in high intensity elite athletes’ training. All DAVID equipment for the spine, shoulder, hip and knee is developed by DAVID’s exercise specialists with biomechanical precision based on several elements:
Correct Posture – the equipment is designed for proper and correct posture (the angle and degrees) to maximize muscle group (agonist) fatigue
Isolation – for optimal training stimuli of the agonist, the antagonist is isolated as much as possible to avoid compensatory behavior.
Resistance Curves – the natural loading principles of the DAVID Resistance Curve incorporates the strength-length, stretch-shortening-cycle and fatigue phenomena uniquely.
Safe training – the natural loading allows safe training without harmfully straining the joints.
Effectiveness – all DAVID equipment is designed to automatically adjust all settings to individual settings.
Isolated vs. functional training
Why is isolated training on DAVID exercise equipment an advantage over functional training? Patients with musculoskeletal complaints compensate for the complaints by using other muscle groups that take over the original movement. This physical compensation is a natural phenomenon but should be restored during rehabilitation.
The DAVID equipment uses isolation to activate muscle groups that the above phenomenon can restore. An example of this is the Hip-Fix on the DAVID Back Extension.1
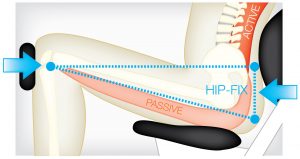
DAVID’s isolation principle
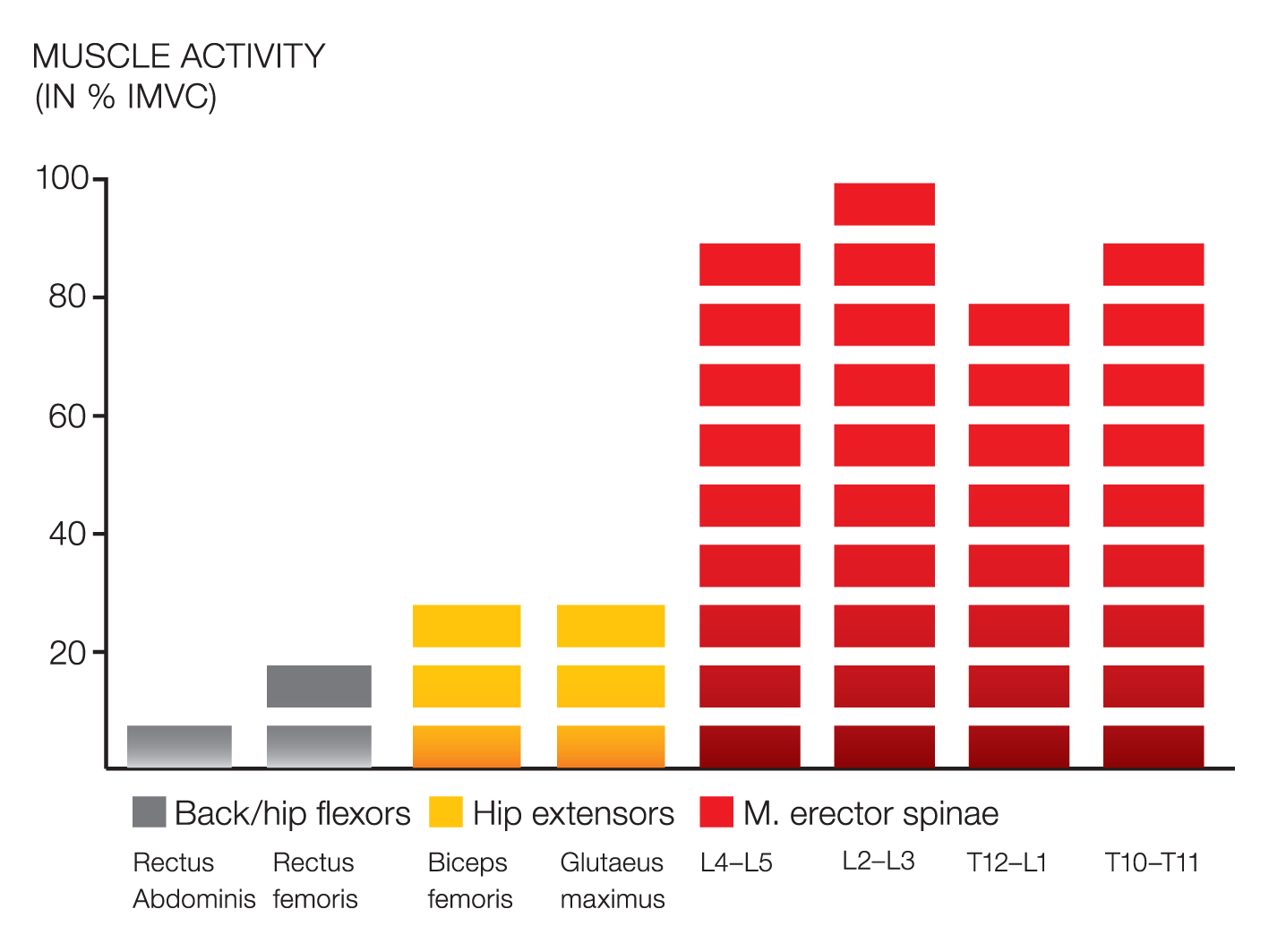
EMG validation studies were conducted in collaboration with the University of Cologne, Germany, to determine if DAVID’s isolation principle activated the target muscles (agonist) and blocked the surrounding solid muscles (antogonist). This example of the Back Extension Device results showed that the strong hip extensors were blocked and inhibited, creating a high activation level in the spinal target muscles.
Enhancing Training Safety with Optimized Resistance Curves
Inadequate resistance curves during training can lead to injuries and discomfort. A prime example of this issue can be observed in specific leg extension machines available in the market. These machines offer excessive light resistance in the initial part of the movement, tempting users to employ momentum to accelerate the motion. This can lead to overloading and be dangerous during the extended position, particularly in the eccentric phase with extended legs. Another typical instance of this, is using rubber bands for glenohumeral joint internal and external rotation exercises. These bands increase resistance when stretched, despite diminishing strength during the movement. This can place undue strain on the joint, resulting in minimal training effectiveness.
To ensure the safety of the muscles and joints, it is crucial to employ proper resistance curves that align precisely with the muscles’ capacity at every movement stage. Additionally, these curves should account for factors such as the stretch-shortening cycle and disproportionate fatigue, which are elaborated further in the “Efficiency” section. Correct resistance curves can optimize training to maximize benefits while minimizing the risk of injury and discomfort.
Ensuring Safe Training with DAVID
At DAVID, safety goes beyond the standard CE-certification considerations. While most devices meet these safety requirements, we prioritize safety by achieving positive outcomes for your body during training, without causing strained muscles or hurting joints. We understand the importance of well-designed equipment to prevent potential harm and injury.
A common issue we’ve observed is with certain back machines found in fitness centers, where the biomechanical features are poorly designed. As a result, users may unknowingly overload certain body parts or perform exercises incorrectly, leading to more harm than good. At DAVID, we are committed to providing solutions that prioritize your well-being and ensure a safe and effective training experience.
Optimizing Joint Alignment for Safe Training
While joint alignment may seem straightforward, it is a complex matter that requires careful consideration. In the design of exercise devices, a common mistake is visually aligning the joint based on its static position, overlooking crucial dynamic factors. In dynamic movements, the center point of the joint can significantly differ from its static location.
Let’s take the shoulder joint’s horizontal abduction or adduction (peck deck) as an example. Current devices in the market position the axis points at shoulder width, but the correct alignment requires the axis points to be approximately 10 cm narrower to each other. The reason behind this lies in the fact that during this exercise, the shoulder joints follow an arching path, and the “virtual joint” is situated between the shoulder joint and the medial connection of the collarbone.
At DAVID, we meticulously analyze and identify the accurate “virtual joint” positions for each exercise device. This approach has enabled us to create exercises that are dynamically safe and comfortable, catering to individuals with various joint and muscle-related concerns. With our emphasis on proper joint alignment, we strive to provide a training experience that promotes safety, effectiveness, and overall well-being.
Optimizing Movement Range for Safety and Versatility
When it comes to movement range, catering to both healthy individuals and those with joint and muscle concerns requires a well-designed and secure device. At DAVID, we prioritize the safety and effectiveness of our equipment to accommodate diverse user needs.
We achieve controlled range of motion through two essential features:
- Range of Motion Adjustment (RMA): This function allows users to adjust the starting point of their movement, ensuring that there is no excessive load beyond a specific point. It offers a tailored approach to suit individual capabilities.
- Range of Motion Control (RMC): Our innovative EVE display showcases clear and colorful zones, with green representing the safe range for movement. Users are encouraged to keep their movements within these zones. If the movement exceeds the safe range, a red alarm signals potential risk, prompting immediate attention.
With the EVE software system, users not only train within the prescribed safe movement range but also develop better movement control. Even if control falters, the RMA mechanisms step in to ensure that the movement remains within a safe and controlled range.
At DAVID, we aim to provide a comprehensive training experience that enhances safety, flexibility, and overall user confidence. Our advanced features empower individuals to exercise comfortably within their capabilities while supporting those with specific joint and muscle concerns.
The Vital Role of Gravity (Inertia) in Training
Scientific evidence highlights the crucial role of gravity-induced resistance in fully engaging muscle cells and achieving optimized training outcomes. Throughout the course of human evolution, our muscles and nervous system have evolved to adapt to inertial loads, making them an essential component of effective training.
Studies have shown that performing concentric contractions of the knee extensor muscles against variable resistance generated by gravity leads to optimal loading conditions. These conditions foster significant fatiguing effects on neuromuscular performance, contributing to enhanced training results.
In summary, gravity’s inherent resistance is indispensable in maximizing the engagement of muscle cells and facilitating superior training outcomes. By capitalizing on this natural force, we can tap into our body’s evolutionary adaptations and unleash the full potential of our training endeavors.
Enhancing Usability for Easy and Effective Training
Our third objective is to design exercise technology that prioritizes user-friendliness, ensuring anyone can use it confidently after basic instructions. Achieving this goal can be challenging due to the need for precise adjustments based on different body types and firm fixations for proper isolation and movement control, which are essential for safety and effectiveness. Typically, rehabilitation devices are so complex that physiotherapists must make multiple adjustments and secure patients into the devices, making it less user-friendly.
DAVID has successfully addressed this challenge through smart technical solutions. We utilize actuators to handle major adjustments based on position information retrieved from the cloud. Minor adjustments are automatic, using gas springs or linearly sliding cushions. Lockable gas springs are employed for body fixations, offering quick, easy, and secure isolation of the target joint. To enhance user experience, all elements that users need to interact with are highlighted in yellow for easy recognition. With our approach, all DAVID devices can be operated by patients without assistance, ensuring correct positions at all times.
Another critical aspect of rehabilitation device usage is to ensure accurate and personalized training. With other exercise therapy devices in the market, numerous variables, such as loading, start and end positions of movements, movement speed, duration, number of sets, and range progressions, need to be done individually and monitored by the patient. Mistakes can quickly occur, even with close supervision by therapists, leading to incorrect compliance.
To address this, DAVID employs clear and colorful terminals that provide visual guidance, enabling patients to follow treatment programs independently and confidently. Our intelligent cloud software delivers up-to-date and location-independent individualized data, allowing for precise control over these training variables. With DAVID, users feel empowered through ease of use, while automatically-collected data provides real-time insights into compliance levels. By prioritizing usability and personalized guidance, we ensure that each user can achieve the correct “dosage” of training for optimal results.
Effectiveness
Prioritizing Effectiveness
At DAVID, our primary goal in equipment design has always been to make exercises as practical and effective as possible within safety limitations. To achieve this, we have dedicated ourselves to extensive research for over a quarter of a century, both participating in and initiating studies in various areas. Our knowledge is built upon decades of biomechanical and physiological research, dating back to the 1980s, and continues with ongoing studies in Finland, Germany, and Austria.
We don’t settle for mere assumptions about having the right technology or concept; instead, we believe in proving the efficacy of our solutions. Our commitment to research may be a long road, but the rewards are worth it. A remarkable example of this is the NICE guidelines in the UK, where our work made a significant impact. It took 17 years from the publication of a study involving DAVID back devices before it was incorporated into the guidelines for treating chronic and recurrent back pain.
At DAVID, we are driven by a relentless pursuit of effectiveness, ensuring that our equipment and concepts are backed by scientific evidence and practical results. By continuously advancing our understanding and staying at the forefront of research, we aim to deliver solutions that genuinely enhance the training experience and contribute to improved health and well-being for all.
The Importance of Specific Training
There is a common belief that exercises with guided movement patterns using exercise devices are less effective than free-weight exercises that enhance overall coordination. However, this notion contains a fundamental error. Each exercise imparts specific skills relevant only to that particular movement and not much more. For instance, a bench press primarily trains the muscles and nervous system to perform that specific movement efficiently, with limited benefits.
We must question whether an exercise is the most effective and safe way to train the chest, shoulder, arms, and corresponding joints. In reality, a reverse correlation exists between the level of coordination required and the intensity of safe training. Exercises demanding higher coordination tend to have lower training intensity, while those with high intensity, like squats, carry a higher risk of injury. Squats can be highly effective at intense levels, but they also pose a significant risk to the back, leading to injuries, especially for young and enthusiastic individuals.
Therefore, it is vital to determine the primary motivation behind each exercise. Is it to become skillful in a specific movement, like bench press, or is it to strengthen the muscles and joints to function effectively in daily life or participate in sports safely? Understanding the purpose of each exercise helps us tailor training regimens that align with specific goals to prioritize overall strength, functionality, and safety.
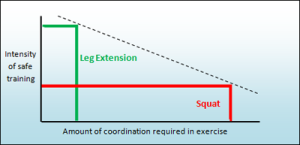
Our philosophy is the latter and therefore our sole motivation is provide safe, effective and comfortable exercises for all relevant muscle groups and joints in the human body.
The Loading Principle in Exercise Equipment Design
When it comes to designing exercise machines, a significant distinction exists between multi-joint and single-joint exercises. In multi-joint exercises like the leg press, load regulation occurs automatically as the human skeletal leverage changes. In contrast, single-joint movements such as the leg extension require precise loading regulations, often accomplished with an eccentric cam. This critical difference means that even without restrictions in loading, a multi-joint training device can be quite effective as long as it functions smoothly and provides adequate seats, grips, and footplates.
However, the same cannot be said for single-joint movements. Many devices available in the market are far from being safe in this regard. While they may carry the CE mark indicating technical safety, little attention is given to biomechanical safety, which is equally crucial for ensuring effective and safe workouts.
At DAVID, we understand the importance of optimizing the loading principle in both multi-joint and single-joint exercises. Our exercise equipment is carefully designed to ensure biomechanical safety and effectiveness, providing users with a secure and productive training experience. By prioritizing both technical and biomechanical safety, we aim to deliver exercise machines that help users achieve their fitness goals while minimizing the risk of injury.
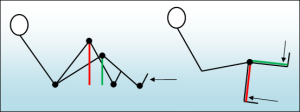
In a multi-joint movement the load is regulated by the changing “leverage arm” of the musculoskeletal system. In the beginning (red) the leverage is long, as legs are straightened (green) the leverage shortens and the load to muscles is reduced. In a single joint movement, the leverage arm remains the same in all positions and therefore the load must be regulated by the device.
Strength – length relationship of the Sarcomere
The first basis for the loading design is the functioning principle of the smallest part of the human muscle, the “Sarcomere”. Sarcomereconsists of two different elements, the ”myosin” and the “actin”. These elements slide within each other and are connected with “cross bridges”.
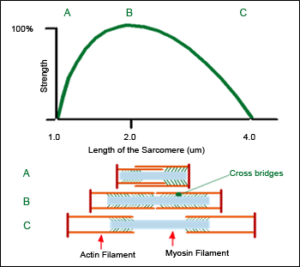
The number of active, interacting cross bridges determines the pulling strength of the sarcomere. The greatest number of interactions occurs at the midpoint of the length of the sacromere. When lengthened, many cross bridges are detached and when shortened, they cross over each other.
In short, purely concentric pulling force of the muscle is the greatest at the midpoint of the movement.
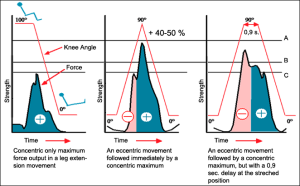
Stretch Shortening Cycle
This graph below shows the importance of eccentric / concentric cycle. Eccentric movement is when muscles are activated while lengthening and concentric movement when muscles are shortening. This diagram shows that muscles strength in the concentric phase is significantly higher when perceived by an eccentric phase (graph in the middle) compared to a situation where there is only concentric phase (graph on the left). This stored energy is lost if the eccentric phase is stopped for 0,9 seconds or more (graph on the right).
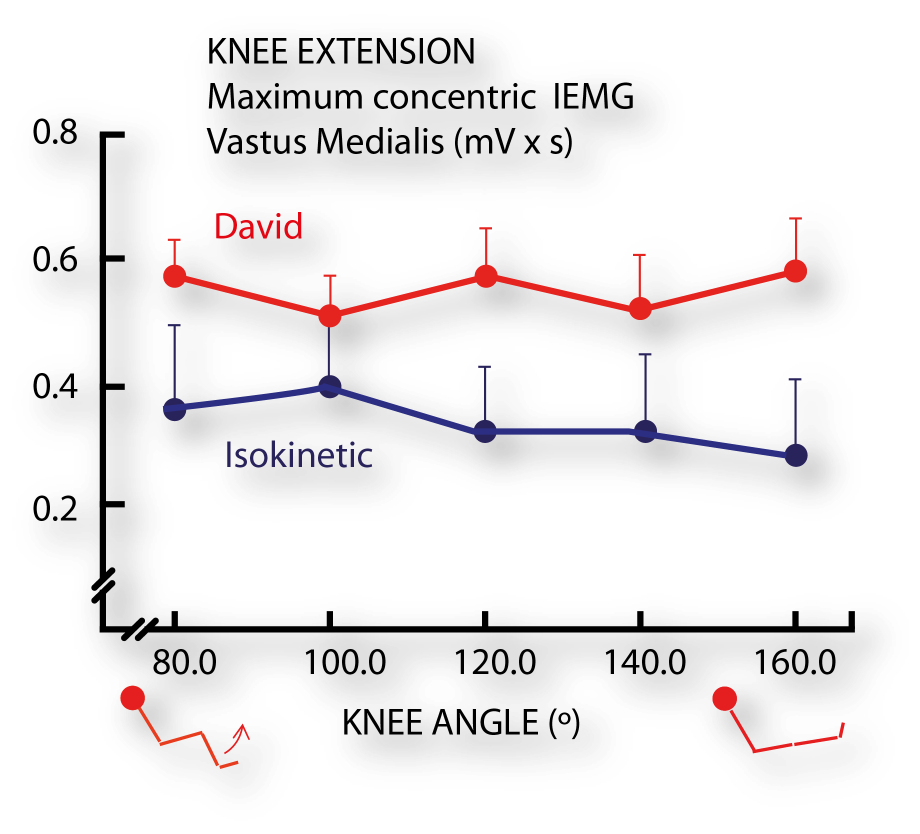
Electromyographic and force production characteristics of leg extensor muscles of elite weight lifters during isometric, concentric, and various stretch-shortening cycle exercises. (4)
This phenomenon is a critical characteristic of the muscles in real world situations where we have to face mass with inertia. Without even understanding this theory, we practice this in every-day situations. For example if we need to jump up, we go down first in a rapid movement before changing direction. This would seem unusual, since we have to first decelerate our body mass on the way down and again accelerate on the way up.
Artificial loading principles, like air pistons or electric motors can never imitate inertia in a proper way. This is a serious drawback, since inertia behaves totally differently during fast or slow movements and “stretch shortening cycle” is a critical component of muscle development. Combined with the David cam principle and natural loading with inertia, David can provide the most effective loading at any speed regardless of the training motivation.
Disproportionate muscle fatigue
Muscle fatigue causes the reduction of strength with each passing repetition. This is a very well known fact and can be felt by every exerciser.
What is not so well known is that the muscle does not fatigue in a proportional manner at all muscle lengths. In fact the muscle fatigues in a disproportional manner in shortened position.
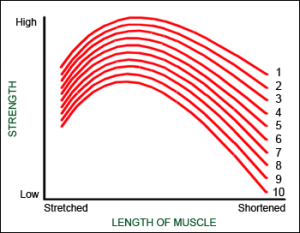
The graph demonstrates that the “strength curve” produced by the muscle changes as the fatigue progresses.
This phenomenon is better understood if we do one single, maximum repetition. Since the load is very high, the movement is very slow. What happens is that during this one repetition, muscle fatigues. So at the end of the movement muscle is more fatigued than in the beginning, which is logical. In the same manner fatiguing happens also with a set of repetitions
Nordic Design by simplicity, minimalism and functionality
The Nordic Design of the devices makes DAVID the best-in-class Exercise Equipment.
The end-user experience is the ultimate driving force for us at DAVID when we design new products. Naturally we have to think about costs, maintenance and other client (facility owner) related matters, but they should never supersede the end user needs.

Introducing DAVID Exercise Equipment – World’s Quietest Devices
In November 2022, DAVID unveiled its revolutionary fully Automatic Weight Selection (AWS) system after extensive development. This cutting-edge automation technology stands out for its silent operation and patient-friendly adjustment method. Gone are the days of bending down to insert weight pins – our devices now set themselves automatically by loading the patient’s settings.
With DAVID’s AWS system, patients can experience seamless automatic weight adjustment, seat height setting, and range of motion adjustment. This level of automation empowers patients to train independently without the need for therapist intervention, all without compromising on the quality of their workouts or the correctness of exercise execution.
Our innovative technology ensures that training remains patient-focused, hassle-free, and noiseless, providing an unmatched training experience for users. At DAVID, we strive to redefine exercise equipment standards, delivering solutions that prioritize convenience, precision, and effectiveness. Experience the world’s most silent devices with DAVID Exercise Equipment today.
Overview studies:
- Denner A. Muskuläre Profile der Wirbelsäule. Berlin, Heidelberg; Springer; 1997. Chapter 7.4, Ergebnisse Eigener Reliabilitäts- und Validitätsuntersuchungen; p. 163–178.
- Häkkinen K, Komi PV, Kauhanen H. Scientific Evaluation of Specific Loading of the Knee Extensors with Variable Resistance, ‘Isokinetic’ and Barbell Exercises. Med Sport Sci. 1987;26:224-237.
- Häkkinen K, Kauhanen H, Komi PV. Effects of fatiguing loading with a variable resistance equipment on neural activation and force production of the knee extensor muscles. Electromyogr Clin Neurophysiol. 1988;28(2-3):79-87.
- Häkkinen K; Komi P V; Kauhanen H., 1989. International journal of sports medicine 1986;7(3):144-51.
Transforming musculoskeletal care
Download our Ebook
 English
English 





















